What is hydrogen bonding?
In nucleic acids:
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond. These bonds can occur between molecules (intermolecularly), or within different parts of a single molecule (intramolecularly).
Intermolecular hydrogen bonding:
Intermolecular hydrogen bonding is seen in water molecule as a result of interaction between the oxygen and hydrogen atom of water molecule. Since oxygen is somewhat electronegative, it acquires electrons of both hydrogen atom towards itself and thus the oxygen atom seem to have negative charge while hydrogen with positive.The hydrogen bond is the result of interaction between these two charges.
 |
| Intermolecular Hydrogen bonding in water molecules |
In the above fig. the dotted lines represents the Hydrogen bond between water molecules.
In nucleic acids:
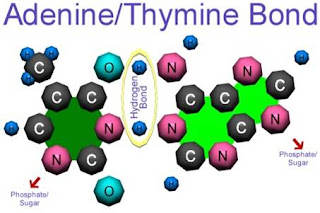 |
In this basepair, adenine and thymine is connected by two hydrogen bonds, as a interaction between the nitrogen, oxygen with hydrogen molecule.These hydrogen bonds between the base pairs plays a major role in all living organisms
Intramolecular hydrogen bonding:
Intramolecular hydrogen bond refers to a hydrogen bond within that particular molecule. It is seen in organic molecules like O-nitrophenol,salicylic acid and salicylaldehyde.
Significance of Hydrogen bond:
- In water molecule, it increases it’s boiling point to 100 degree celsius and hence makes the water to sustain in high temperatures.
- It plays a major role in nucleic acids, which acts as a nucleus to all living organisms.


No comments:
Post a Comment